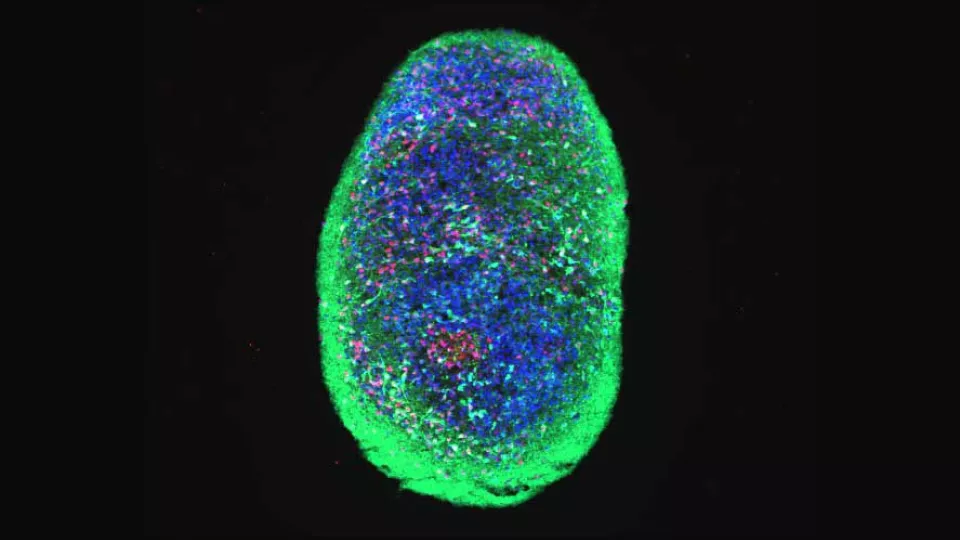
Parkinson’s disease (PD) is a neurodegenerative disorder linked to pathological aggregation of the protein alpha-synuclein affecting the midbrain regions involved in movement regulation. The aggregation process has been thoroughly studied. Still, what is really causing the aggregation remains largely unknown.
To elucidate what goes wrong inside patient brain cells, Laurent Roybon and colleagues used skin biopsies from Parkinson’s patients and healthy controls. They managed to convert these skin cells, via induced pluripotent stem cells, into midbrain cells resembling those present in the brain of the individuals. And they noticed that the brain cells derived from individuals with Parkinson’s disease started to accumulate pathological amounts of alpha-synuclein.
“Our data suggest that changes in the milieu inside the cells induced by patient genomes trigger structural changes of alpha-synuclein. This potentially leads to the formation of aggregates having different structures, pathogenicity, and seeding propensities,” tells Laurent Roybon, last author of the study.
Using a specific assay, they discovered that the content from patient-derived brain cells modulated alpha-synuclein aggregation.
What was unexpected was to find that the cellular milieu of patients without known genetic causes of the disease also modulated the aggregation of alpha-synuclein.
“What was unexpected was to find that the cellular milieu of patients without known genetic causes of the disease also modulated the aggregation of alpha-synuclein. This data is crucial as it suggests that the pathological changes in these patients may have an underlying (epi)genetic pathological process. Therefore, employing brain cells converted from patient skin cells could inform us on the disease which is represented by 90% of PD cases where genetic causes are not known,” explains Laurent Roybon.
Future studies will help the researchers to determine what cellular components within patient brain cells trigger the structural changes of alpha-synuclein that leads to its aggregation. This could lead to the development of therapeutic strategies to prevent alpha-synuclein aggregation, and thereby disease progression.