September is Alzheimer’s awareness month. Around the globe, over 30 million people are estimated to have the disease. And the number is increasing due to an aging population. Alzheimer's disease is characterized by the abnormal accumulation and spread of the two proteins tau and amyloid-beta in the brain. Despite intensive research and expensive clinical trials, there is still no cure. Unfortunately, when the patients get their proper diagnosis, the pathological changes have already evolved over decades, making the damage hard to rescue. But recent discoveries and development of diagnostic tools from MultiPark’s research groups bring back hope.
Four distinct subtypes of Alzheimer’s disease
Professor Oskar Hansson, the vice coordinator of MultiPark, is in charge of the BioFINDER cohorts, conducted by researchers at LU and Skåne University Hospital. The Swedish BioFINDER study is a goldmine for clinical research on biomarkers for early and safe diagnosis of Alzheimer’s disease. Including over 3,000 healthy controls and patients with neurodegenerative diseases, such as Alzheimer's disease, the cohort is unique in its kind. Earlier this year, two impactful discoveries have been made by MultiPark researchers using BioFINDER.
In April, an international study including BioFINDER revealed that the Alzheimer’s protein tau spreads in four distinct patterns. Each pattern also leads to diverse symptoms and different prognoses for the patients.
Algorithm predicting Alzheimer’s
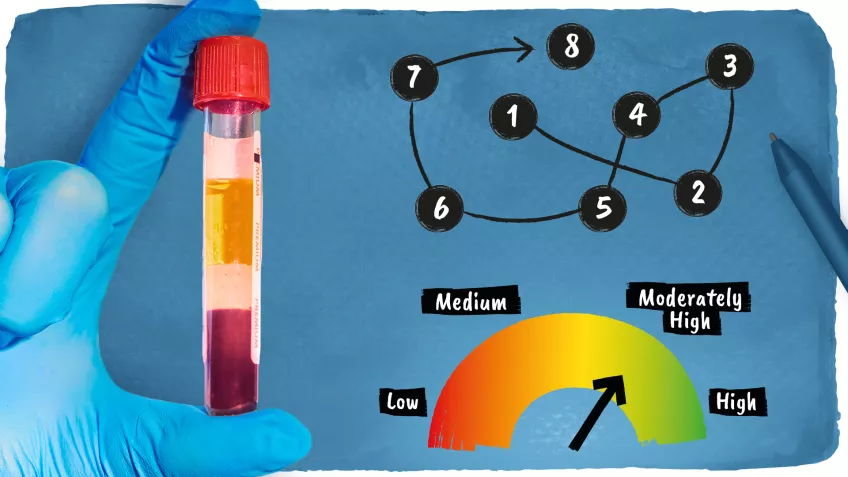
One month later, in May, the same cohort was used to develop an algorithm combining data from a simple blood test with brief memory tests, to predict with over 90% certainty who will develop Alzheimer's disease. The blood test measures a variant of the tau protein and a risk gene for Alzheimer’s and the cognitive test only takes 10 minutes to complete.
” We have now developed a prototype online tool to estimate the individual risk of a person with mild memory complaints developing Alzheimer's dementia within four years”, tells Sebastian Palmqvist, first author of the study and associate professor at Lund University.
Transdisciplinary collaboration to improve health-care for patients with Alzheimer’s
Based on findings from the BioFINDER study, nine senior researchers from five different faculties at Lund University have initiated a transdisciplinary initiative to improve the early diagnosis of Alzheimer’s disease. Four of them are research leaders from MultiPark, whereof Oskar Hansson coordinates the collaboration. Other contributors are Region Skåne, Lund Municipality, and the diagnostic and pharma industry.
“In the last year we have had great breakthroughs when it comes to blood-based biomarkers for Alzheimer’s disease. Now we will develop accurate and cost-effective diagnostic and prognostic algorithms that can be used in both clinical practice and trials,” explains Oskar Hansson.
“We are very grateful for this support; it will allow us to create a platform for co-producing knowledge and identifying collaborative projects,” adds Maria H Nilsson, vice coordinator of the initiative and one of the research leaders from health sciences at MultiPark.
Moreover, simple diagnostic tools could also open new opportunities for drug development, as it facilitates the recruitment of people with Alzheimer's at an early stage then drug candidates still have a chance of delaying or preventing the disease development. But, to find new pharmacological targets, unraveling the early pathological changes at the molecular level is crucial. And here comes the strength of Multipark as a multidisciplinary research environment. During the summer, two important discoveries have been made by MultiPark’s experimental scientists.
Detection of early amyloid-beta accumulation inside neurons
In mid-July, researchers from the Experimental Dementia Research group revealed that amyloid-beta accumulates inside neurons and that the misfolded protein may thereafter propagate from one neuron to another. And this happens before the aggregation into so-called amyloid-beta plaques in the brain.
“The plaques of amyloid-beta outside the nerve cells have long been a target for treatment of Alzheimer’s disease. But as treatments to remove plaque have not helped against dementia, we must develop and investigate other hypotheses in order to find other targets for treatment. Our results indicate that we must focus on misfolded amyloid-beta inside the nerve cells that arise far earlier than the visible plaques”, says the first author of the study Tomas Roos, a doctoral student in one of MultiPark’s research groups and resident physician at Skåne University Hospital’s neurological clinic.
Hence, being able to observe the structural changes of molecules as amyloid-beta may be the key to understand what goes wrong. But up to date, knowledge in how to capture this process visually has been scarce.
A new approach to follow structural changes of Alzheimer’s proteins inside the neuron
About a week later, another of MultiPark’s more than 40 senior researchers offered a solution to this problem. The Medical Microspectroscopy group demonstrated how a novel imaging approach can be used to achieve structural and chemical information within a single neuron.
“With our approach, we capture elemental distribution and fibrillary forms of amyloid-β proteins within the same neuron at sub-cellular resolution”, explains Oxana Klementieva, leading the Medical Microspectroscopy group at MultiPark and last author of the study.
The secret of getting this detailed structural information is to combine already available techniques that stand at the opposite ends of the electromagnetic spectrum. Together, they are a perfect match. Using hard X-rays, metal ions can be traced with the range of nanometers, while using the infrared part of the spectrum, it is possible to assess molecular structures. With these techniques, it is possible to trace elements and relevant molecular structures directly in neurons. Hence, ongoing research projects at MultiPark raise the expectations of being closer than ever before to unravel the puzzle of Alzheimer’s disease, piece by piece.